An Atom With a Net Positive Charge Must Have More
An atom is defined as having the same number of electrons negative charge protons. An atom having fewer protons than electrons carries a net negative charge and is called an _____.

Quarked How Did The Different Types Of Quarks Get Their Names Fun Science Quantum Mechanics Science Facts
If the atom gains an electron there will be more electrons and will have a negative.

. When we know that in Adam comes out with a positive charge it has to have more protons than electrons. The only way for a neutral atom to become positive is by the removal of an electron. A more protons than electrons B more electrons than protons C more neutrons than protons D more protons than neutrons.
Net positive charge pulls e- in tighter. And 2 more electron-electron repulsion. Ionic bonds arise from.
By adding one more electron we get a negatively charged Cl-ion with a net charge of -1. Usually these two numbers are equal so the atom is neutral charge of zero. If an atom gains an electron then it has more electrons which gives it a negative charge.
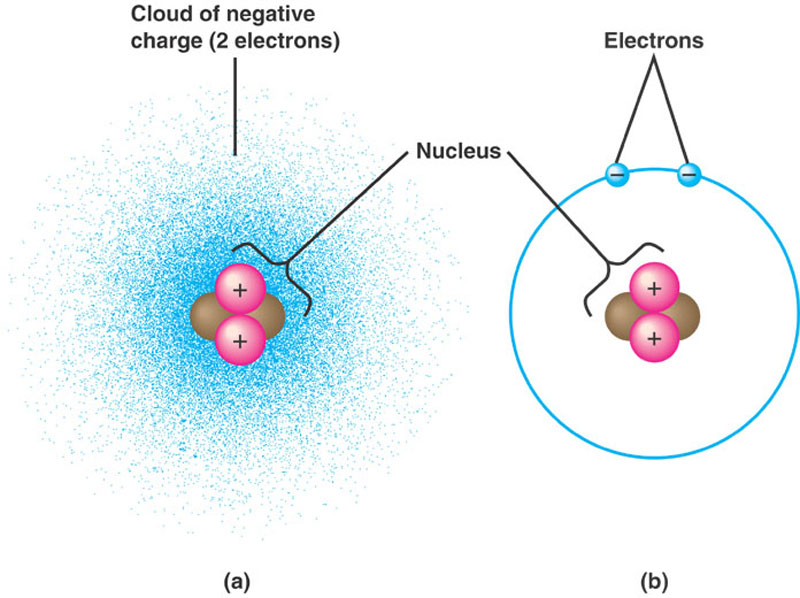
An atom having more protons than electrons has a net positive charge and is called a _____. A neutral chlorine atom for example contains 17 protons and 17 electrons. Sometimes ions occur that have more.
The number of neutrons. If the number of electrons and protons are the same then the net charge is zero. More protons than electrons.
Since protons have positive charges and electrons have negative charges an ion with. The isotopes carbon-12 and carbon-14 differ in. Heating the reactants increases the rate of a reaction because the reactants collide with one another more often.
The charge on an atom depends on the number of protons and electrons present in it. By removing an electron from this atom we get a positively charged Na ion that has a net charge of 1. The reason is the atom now has more protons positive charges than electrons negative charges.
Care must be taken so molecules arent destroyed Temperature. Monatomic ions are formed from single atoms while polyatomic ions are formed from two or more atoms that have been bonded together in each case yielding an ion with a positive or. If an atom loses an electron then it has more protons which makes it positively charged.
An atom with a net positive charge must have more. B Why are monatomic anions larger than their corresponding neutral atoms. If an atom has one valence electron that is a single electron in its outer energy level it will most likely form Ionic Bond An atom with a net positive charge must have more.
Ions with a positive charge are referred to as a cationWhat is the charge of an ion if the atom gains an electron. The charge of an atom is the number of protons minus the number of electrons. And 3 less electron-electron repulsion.
An atom with the same number of protons and electrons has no overall charge so if an atom loses the negatively charged electron it will have more protons therefore a positive charge. An ion is an atom with a positive or negative charge because the atom either gained or lost an electron. For example sodium and A becomes positive when it donates its.
For a neutral atom the number of protons balances the number of electrons. Any atom will have a net positive charge if it loses electrons the negative particles in the atom. Protons in the nucleus than there are electrons outside the nucleus.
Since electrons have a negative charge if there are more electrons than the atom cannot be positively charged. The number of protons. Atoms in which the number of electrons does not equal the number of protons are known as _____ and they are charged particles.
The protons in an atom are positively charged while electrons have a negative charge. Anions are larger than their parent atom because 1 net negative charge allows electrons to wander farther out. In order to have a positive charge an atom must have _____.
For a negatively charged atom the number of electrons is more than the number of protons. An ion is an atom or group of atoms that has lost one or more electrons giving it a net positive charge cation or that has gained one or more electrons giving it a net negative charge anion. More protons positive charges will have.
If the number of protons is bigger than the number of electrons the atom has a net positive charge and if the number of electrons is bigger than the number of protons the atom has a net. Atoms that gain extra electrons become negatively charged. An atom with a net positive charge must have.
More protons than electrons The hydrogen and oxygen atoms of a water molecule are held together by ________ bonds.

Heat Capacity Specific Heat Capacity Bundle Covalent Bonding Scaffolded Notes Lab Activities

The Activation Energy Is The Amount Of Energy Required For A Reaction To Occur When A Catalyst Enzyme Interacts With Energy Activities Active Site Activities
No comments for "An Atom With a Net Positive Charge Must Have More"
Post a Comment